Multiple Choice
What is the oxidation state of the underlined atom in the reaction: 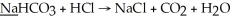
A) 0
B) +1
C) -1
D) +2
E) -2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q76: The oxidation number of P in PO<sub>4
Q77: From the activity list included in this
Q78: Which battery system is based on half
Q79: What is the oxidation state of the
Q80: From the activity list included in this
Q82: Balance the following half reaction in base
Q83: Oxidation typically involves:<br>A)the loss of electrons.<br>B)the loss
Q84: Identify the oxidizing agent in the following
Q85: Using the activity list included in this
Q86: The half-reaction 2 Br <sup>1-</sup> → Br<sub>2</sub>