Multiple Choice
What is the oxidation state of the underlined atom in the reaction: 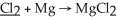
A) 0
B) +2
C) -2
D) +4
E) -4
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q67: What is the oxidation state of sulfur
Q68: Distinguish between a galvanic (voltaic)cell and an
Q69: What is the oxidation state of the
Q70: The oxidizing agent typically:<br>A)loses electrons.<br>B)gains oxygen.<br>C)is the
Q71: Gold metal is found at the bottom
Q73: Which fact about fuel cells is FALSE?<br>A)Fuel
Q74: Most acids dissolve metals by the oxidation
Q75: Electrons flowing through a wire is an
Q76: The oxidation number of P in PO<sub>4
Q77: From the activity list included in this