Multiple Choice
What is the balanced oxidation half-reaction for the following unbalanced redox reaction: 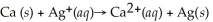
A) Ca → 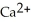 + 2
+ 2 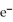
B) 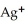 → Ag +
→ Ag + 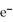
C) 2 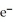 + Ca →
+ Ca → 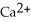
D) 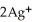 + 2
+ 2 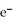 → 2Ag
→ 2Ag
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: The substance being reduced is the oxidizing
Q7: In electrolysis:<br>A)a spontaneous redox reaction produces electricity.<br>B)a
Q8: A spontaneous redox reaction can be used
Q9: Reduction is the gain of electrons.
Q10: An electrochemical cell is based on the
Q12: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6110/.jpg" alt=" (aq)+ 2Na (s)→
Q13: The oxidizing agent is the substance being
Q14: Assign the oxidation state of each atom
Q15: Flashlight batteries are called dry cells.
Q16: For the reaction Co + Cl<sub>2</sub> →