Multiple Choice
What is the balanced reduction half-reaction for the following unbalanced redox reaction: 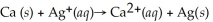
A) Ca → 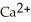 + 2
+ 2 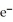
B) 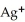 → Ag + 1
→ Ag + 1 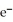
C) 2 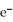 + Ca →
+ Ca → 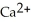
D) 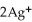 + 2
+ 2 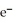 → 2Ag
→ 2Ag
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q61: From the activity list included in this
Q62: The oxidation number of manganese in MnO<sub>4</sub><sup>1-</sup>
Q63: From the activity list included in this
Q64: The oxidation number of Cr in Cr<sub>2</sub>O<sub>7</sub>
Q65: Redox reactions must involve the gain or
Q67: What is the oxidation state of sulfur
Q68: Distinguish between a galvanic (voltaic)cell and an
Q69: What is the oxidation state of the
Q70: The oxidizing agent typically:<br>A)loses electrons.<br>B)gains oxygen.<br>C)is the
Q71: Gold metal is found at the bottom