Multiple Choice
Balance the following half reaction in acid solution: 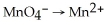 (aq)
(aq)
A) 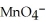 →
→ 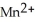 + 3
+ 3 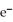
B) 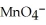 + 8
+ 8 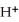 →
→ 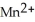 + 4
+ 4 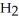 O
O
C) 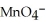 + 8
+ 8 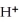 →
→ 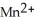 + 4
+ 4 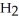 O +5
O +5 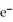
D) 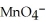 + 8
+ 8 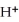 + 5
+ 5 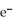 →
→ 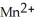 + 4
+ 4 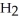 O
O
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q53: It can be shown that copper metal
Q54: What is the oxidation state of the
Q55: Using the activity list included in this
Q56: A sacrificial electrode works by being reduced
Q57: In the reaction S + O<sub>2 </sub>→
Q59: From the activity list included in this
Q60: In an electrochemical cell,which of the following
Q61: From the activity list included in this
Q62: The oxidation number of manganese in MnO<sub>4</sub><sup>1-</sup>
Q63: From the activity list included in this