Multiple Choice
Given the following diagram,describe what happens electronically between these two molecules. 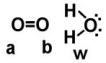
A) Oxygen A becomes slightly positively charged due to the protons on the water molecule.
B) Oxygen B becomes slightly positively charged due to the protons on the water molecule.
C) Oxygen A becomes slightly negatively charged due to the oxygen molecule.
D) Hydrogens on oxygen W becomes slightly positively charged due to the oxygen molecule.
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Q96: What property of alloys make them ideal
Q97: Which should be larger,the potassium atom,K,or the
Q98: If the following generic atom were to
Q99: Which of the following statements describes a
Q100: Classify the following bonds as ionic,covalent,or neither
Q102: Which of the above substances would have
Q103: What is the compound that forms if
Q104: Which of the following is the weakest?<br>A)a
Q105: Which of the following statements is untrue?<br>A)Covalent
Q106: Which of the following elements has six