Multiple Choice
Dipole-induced dipole forces of attraction exist between water and gasoline,and yet these two substances do not mix because water has such a strong attraction for itself.Which of the following compounds might best help to make these two substances mix into a single liquid phase? 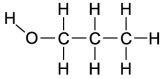
A) the molecule on the far left because the O-H bond is polar and the carbon and hydrogen bonds are nonpolar
B) the molecule in the middle because when the salts mix into the water,it will help separate the water and decrease the attraction for itself
C) The molecule on the right will form attractions with the polar ends of the water,allowing the gasoline a chance to mix with the water.
D) All of these molecules would be equally effective at increasing the mixing of gasoline and water.
Correct Answer:

Verified
Correct Answer:
Verified
Q19: What is the main difference between a
Q20: How many nonbonding pairs of electrons are
Q21: Which of the following is the main
Q22: If an ionic bond is stronger than
Q23: Which of the following molecules contains an
Q25: Which of the following is the correct
Q26: Which of the following is most likely
Q27: Metals are often used for making designer
Q28: Which of the following elements will most
Q29: Which of the following describes how a