Multiple Choice
Consider the boiling points of the following compounds and their solubilities in room-temperature water.Why does the solubilities in water go down as the boiling points of these alcohols go up. 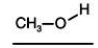

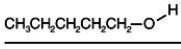
A) Larger molecules are less attracted to one another by induced dipole-induced dipole as well as by dipole-dipole and dipole-induced dipole attractions.
B) As the boiling increases,it is more difficult to keep the alcohol from evaporating out of solution.
C) As the boiling point increases,the size of the alcohol molecules decreases.
D) Larger molecules are more attracted to one another by induced dipole-induced dipole as well as by dipole-dipole and dipole-induced dipole attractions.
Correct Answer:

Verified
Correct Answer:
Verified
Q54: Which of the following would have the
Q55: A substance consisting of which molecule shown
Q56: Which of the following molecules is the
Q57: How many covalent bonds would the following
Q58: Distinguish between a metal and a metal-containing
Q60: In terms of the periodic table,is there
Q61: List the following bonds in order of
Q62: Which of the following statements best describes
Q63: How many oxide ions (O<sup>-2</sup>)are needed to
Q64: Which would you expect to have a