Multiple Choice
The boiling point of 1,4-butanediol is 230°C.Would you expect this compound to be soluble or insoluble in room-temperature water? 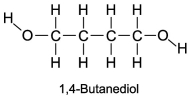
A) Since there are no polar areas on this molecule,it is insoluble in water at room temperature.
B) A high boiling point means that the substance interacts with itself quite strongly.Therefore this molecule is not soluble in water.
C) Since there are polar areas on this molecule,it is insoluble in water at room temperature.
D) Water would be attracted to both ends of 1,4 butanediol,and it is infinitely soluble in water.
Correct Answer:

Verified
Correct Answer:
Verified
Q63: How many oxide ions (O<sup>-2</sup>)are needed to
Q64: Which would you expect to have a
Q65: Which of the following molecules is most
Q66: Which molecule is most polar?<br>A)S=C=S<br>B)O=C=O<br>C)O=C=S<br>D)These all have
Q67: The neon atom tends not to gain
Q69: If the following generic atom were to
Q70: Which of the following would be a
Q71: Which of the following elements has two
Q72: There is more gold in 1 km<sup>3</sup>
Q73: Atoms of nonmetallic elements form covalent bonds,but