Multiple Choice
Two chemical structures are shown,one of a typical gasoline molecule and the other of a typical motor oil molecule.Which is which? 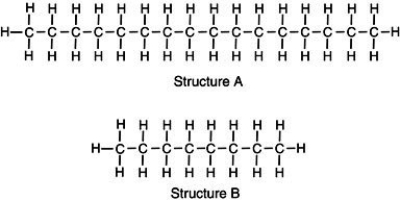 Base your reasoning not on memorization but rather upon what you know about molecular interactions and the various physical properties of gasoline and motor oil.
Base your reasoning not on memorization but rather upon what you know about molecular interactions and the various physical properties of gasoline and motor oil.
A) Structure A represents the gas molecule because there are more bonds to gain energy from,giving it a higher energy content than oil.
B) Structure A represents motor oil,illustrating a molecule with greater induced dipole-induced dipole molecular interactions thus,the molecules are strongly attracted to one another.
C) Structure B represents the oil molecule.Because oil molecules are smaller,they can compact closer together,giving the appearance of a thicker solution than gasoline.
D) Structure B represents crude oil which is processed to generate longer molecules of gasoline to prevent toxic vapors from harming consumers.
Correct Answer:

Verified
Correct Answer:
Verified
Q27: Metals are often used for making designer
Q28: Which of the following elements will most
Q29: Which of the following describes how a
Q30: Which of the following intermolecular forces best
Q31: Which of the following molecules would you
Q33: Which of the following does not describe
Q34: What is happening at the molecular level
Q35: Which of the following is the strongest?<br>A)a
Q36: Take money away from your bank account
Q37: Which of the following compounds has polar