Multiple Choice
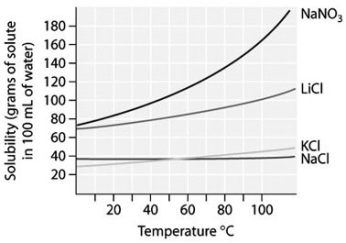
-At 10°C,which is more concentrated-a saturated solution of sodium nitrate,NaN ,or a saturated solution of sodium chloride? (See figure shown above.)
A) At 20°C a saturated solution of sodium nitrate,NaN ,is more concentrated than a saturated solution of sodium chloride,NaCl.
B) At 10°C a saturated solution of sodium chloride,NaCl,is more concentrated than a saturated solution of sodium nitrate,NaN .
C) At 10°C a saturated solution of sodium nitrate,NaN ,is more concentrated than a saturated solution of sodium chloride,NaCl.
D) At 25°C a saturated solution of sodium nitrate,NaN
Correct Answer:

Verified
Correct Answer:
Verified
Q66: How is the solubility of a solid
Q67: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6597/.jpg" alt=" -Which
Q68: If the solubility of a compound is
Q69: Allowing water to cascade or bubble in
Q70: What do chicken noodle soup and garden
Q72: How many grams of sugar (sucrose)are there
Q73: What happens if you were to place
Q74: Deuterium oxide, <span class="ql-formula" data-value="\mathrm
Q75: What is the molarity of 0.5 liters
Q76: Why are noble gases infinitely soluble in