Multiple Choice
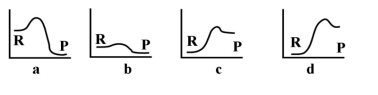
-Given the following energy profiles,which of the following reactions is endothermic? 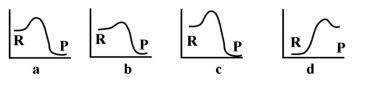 R= reactants P = products
R= reactants P = products
A) a
B) b
C) c
D) d
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q73: What coefficients balance the following equation?
Q74: What is an endothermic reaction?<br>A)It is a
Q75: How does formula mass differ from atomic
Q76: Balance these equations. _ <span
Q77: Wild plants readily grow "all by themselves"
Q79: Balance the following chemical equation. _ N<sub>2</sub>
Q80: Since some of the compounds that are
Q81: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6597/.jpg" alt=" -For the above
Q82: How many grams of water can be
Q83: What is the mass of an oxygen