Multiple Choice
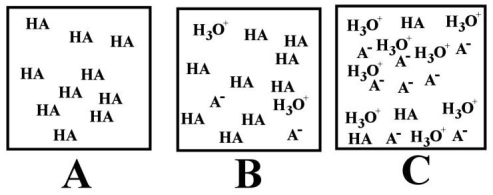
-Which of the above images best depicts a water solution of a weak acid (represented by HA) ?
A) A
B) B
C) C
D) All of the above are weak acids.
E) None of the above are weak acids.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q13: Which of the following statements best describes
Q14: Which of the following statements about strong
Q15: If you had a 1 M solution
Q17: Rust has a tendency to form when
Q19: Iron atoms have a greater tendency to
Q20: What is the relationship between the hydroxide
Q21: What happens to the pH of soda
Q22: What is a base?<br>A)anything that accepts a
Q23: For the following acid-base reaction,identify what is
Q140: For the following reaction,identify whether the compound