Multiple Choice
The phosphoric acid salt of caffeine has the structure: 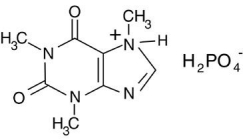 This molecule behaves as an acid in that it can donate a hydrogen ion,created from the hydrogen atom bonded to the positively charged nitrogen atom.What salt is formed when 1 mole of this salt reacts with 1 mole of sodium hydroxide,NaOH,a strong base?
This molecule behaves as an acid in that it can donate a hydrogen ion,created from the hydrogen atom bonded to the positively charged nitrogen atom.What salt is formed when 1 mole of this salt reacts with 1 mole of sodium hydroxide,NaOH,a strong base?
A) Salts can't react to form salts,rather they only arise from the reaction of an acid and a base.
B) P -
C) HP
D) P
Correct Answer:

Verified
Correct Answer:
Verified
Q58: What happens when you use a monomer
Q59: How are monomers and polymers related?<br>A)A monomer
Q60: Would you expect polypropylene to be denser
Q61: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6597/.jpg" alt=" -Which of the
Q62: Which of the following molecules is not
Q64: Formaldehyde is a toxic preservative with the
Q65: An amino acid is an organic molecule
Q66: What is indicated by a gasoline's octane
Q67: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6597/.jpg" alt=" -Which of the
Q68: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6597/.jpg" alt=" -Which of the