Multiple Choice
The coordination compound shown below has 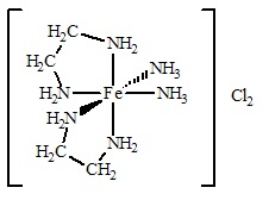
A) two monodentate ligands and two bidentate ligands.
B) three monodentate ligands and two bidentate ligands.
C) four monodentate ligands and two bidentate ligands.
D) one monodentate ligand and four bidentate ligands.
E) four bidentate ligands.
Correct Answer:

Verified
Correct Answer:
Verified
Q80: The correct name of the complex ion
Q81: The ion [Co(NH<sub>3</sub>)<sub>6</sub>]<sup>2+</sup> is octahedral and high
Q82: The electron configuration of a Ti atom
Q83: How many geometric isomers are possible for
Q84: The neutral monodentate ligand L forms the
Q86: Consider the [CoCl<sub>6</sub>]<sup>4-</sup> ion. Determine which responses
Q87: Which one of these complex ions would
Q88: How many unpaired electrons does the manganese
Q89: Which of the following choices can function
Q90: In the complex ion [Co(en)<sub>2</sub>Br<sub>2</sub>]<sup>+</sup>, the oxidation