Multiple Choice
The best name for the complex shown below is 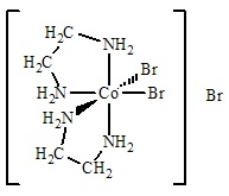
A) cobalt(III) bis(ethylenediamine) bromide.
B) dibromobis(ethylenediamine) cobalt(III) bromide.
C) dibromidedi(ethylenediamine) cobalt(III) bromide.
D) dibromodiethylenediaaminecobalt(III) bromide.
E) tribromobis(ethylenediamine) cobalt(III) .
Correct Answer:

Verified
Correct Answer:
Verified
Q69: What is the coordination number of cobalt
Q70: Labile complexes are coordination complexes that<br>A) are
Q71: How would you expect the molecule 1,10-phenanthroline
Q72: In the complex ion [ML<sub>6</sub>]<sup>n+</sup>, M<sup>n+</sup> has
Q73: Ethylenediaminetetraacetic acid (EDTA) is<br>A) not useful as
Q75: The number of geometrical isomers and optical
Q76: The correct formula for dicarbonylsilver (I) ion
Q77: Predict the number of unpaired electrons in
Q78: The correct formula for the dichlorobis(ethylenediamine)chromium(III) ion
Q79: In the complex ion [Cr(C<sub>2</sub>O<sub>4</sub>)<sub>2</sub>(H<sub>2</sub>O)<sub>2</sub>]<sup>-</sup>, the oxidation