Multiple Choice
Which of these electron energy level patterns would absorb light with the shortest wavelength 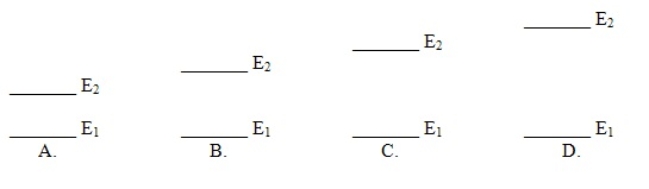
A) A
B) B
C) C
D) D
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: What is the oxidation number of Fe
Q4: The correct name of the complex ion
Q5: Which of the following complexes has optical
Q6: How many unpaired electrons are there in
Q7: Assuming a coordination complex is formed with
Q9: In the complex ion [ML<sub>6</sub>]<sup>n+</sup>, M<sup>n+</sup> has
Q10: The total number of electrons in the
Q11: In the complex ion [ML<sub>6</sub>]<sup>n+</sup>, M<sup>n+</sup> has
Q12: The terms that describe the geometric isomers
Q13: In the coordination compound K<sub>2</sub>[Co(en)Cl<sub>4</sub>], the coordination