Multiple Choice
Determine
(i) the oxidation number of the metal,
(ii) the number of d electrons,
(iii) the coordination number,
(iv) the charge of the complex ion, and
(v) the number and type of ligands for the coordination compound shown below. 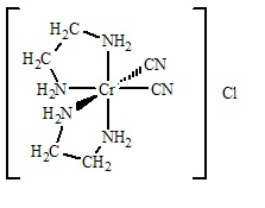
A) i = +2; ii = 3 d electrons; iii = 5; iv = 1+; v = zero monodentate and four bidentate
B) i = +3; ii = 2 d electrons; iii = 6; iv = 1+; v = two monodentate and two bidentate
C) i = +2; ii = 3 d electrons; iii = 5; iv = 1+; v = two monodentate and one bidentate
D) i = +3; ii = 3 d electrons; iii = 6; iv = 1+; v = two monodentate and two bidentate
E) None of the above have all five answers correctly presented
Correct Answer:

Verified
Correct Answer:
Verified
Q17: In the coordination compound [Co(en)<sub>2</sub>Cl<sub>2</sub>]Cl, the coordination
Q18: Which of these ligands produces the weakest
Q19: Which of the following complexes has optical
Q20: What is the oxidation number of Co
Q21: In the coordination compound [Pt(NH<sub>3</sub>)<sub>2</sub>Cl<sub>2</sub>], the coordination
Q23: The electron configuration of a Cr<sup>3+</sup> ion
Q24: In K<sub>4</sub>[Fe(CN)<sub>6</sub>], how many 3d electrons does
Q25: What is the coordination number of chromium
Q26: In Na<sub>3</sub>[Ni(SCN)<sub>5</sub>], how many 3d electrons does
Q27: What is the oxidation number of Co