Multiple Choice
The isomerization of cyclopropane to form propene is a first-order reaction. 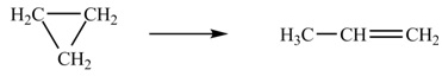 At 760 K, 85% of a sample of cyclopropane changes to propene in 79.0 min. Determine the rate constant for this reaction at 760 K.
At 760 K, 85% of a sample of cyclopropane changes to propene in 79.0 min. Determine the rate constant for this reaction at 760 K.
A) 3.66 * 10-2 min-1
B) 1.04 * 10-2 min-1
C) 2.42 min-1
D) 2.06 * 10-3 min-1
E) 2.40 * 10-2 min-1
Correct Answer:

Verified
Correct Answer:
Verified
Q69: The rate determining step in the
Q70: Which of the following elementary steps
Q71: For the chemical reaction system described by
Q72: A reaction was experimentally determined to follow
Q73: At a certain temperature, the data
Q75: A nuclear stress test utilizes a gamma-emitting
Q76: The following mechanism has been suggested
Q77: The isomerization of cyclopropane to form propene
Q78: For the reaction X + Y
Q79: Aspirin, C<sub>9</sub>H<sub>8</sub>O<sub>4</sub>, slowly decomposes at room