Multiple Choice
The isomerization of cyclopropane to propene follows first-order kinetics. 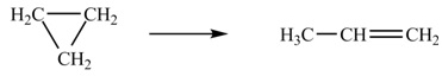 At 700 K, the rate constant for this reaction is 6.2 * 10-4 min-1. How many minutes are required for 10.0% of a sample of cyclopropane to isomerize to propene
At 700 K, the rate constant for this reaction is 6.2 * 10-4 min-1. How many minutes are required for 10.0% of a sample of cyclopropane to isomerize to propene
A) 16,100 min
B) 170 min
C) 3,710 min
D) 1.43 * 10-3 min
E) 1,120 min
Correct Answer:

Verified
Correct Answer:
Verified
Q12: The isomerization of cyclopropane follows first order
Q13: For the reaction BrO<sub>3</sub><sup>-</sup> + 5Br<sup>-</sup>+
Q14: A certain first-order reaction A
Q15: Benzoyl chloride, C<sub>6</sub>H<sub>5</sub>COCl, reacts with water to
Q16: The following mechanism has been suggested
Q18: Chlorine dioxide reacts in basic water
Q19: At a certain temperature, the data
Q20: If E<sub>a</sub> for a certain biological
Q21: The first-order decomposition of SO<sub>2</sub>Cl<sub>2</sub> to
Q22: The following initial rate data apply