Multiple Choice
At 700 K, the rate constant for the following reaction is 6.2 * 10-4 min-1. 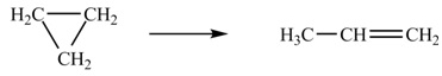 How many minutes are required for 20% of a sample of cyclopropane to isomerize to propene
How many minutes are required for 20% of a sample of cyclopropane to isomerize to propene
A) 1,120 min
B) 360 min
C) 3710 min
D) 1.4 * 10-4 min
E) 280 min
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q32: For the following exothermic reaction, the
Q33: The rate determining step must be the
Q34: A reaction is experimentally found to follow
Q35: An experimental drug, D, is known to
Q36: Nitric oxide reacts with chlorine to
Q38: Complete the following statement: A catalyst<br>A) increases
Q39: The activation energy for the following
Q40: The oxidation of iodide ions by
Q41: The isomerization of methyl isocyanide, CH<sub>3</sub>NC
Q42: For the reaction X<sub>2</sub> + Y