Multiple Choice
The graphs below all refer to the same reaction. What is the order of this reaction 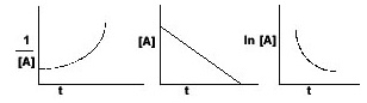
A) zero order
B) first order
C) second order
D) unable to predict
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: For the chemical reaction system described by
Q4: Appropriate units for a second-order rate constant
Q5: For the first-order reaction 2N<sub>2</sub>O<sub>5</sub>
Q6: The half life for a first order
Q7: The activation energy for the reaction
Q9: Concerning the rate law, Rate = k[A][B][C],
Q10: A certain reaction, reaction A <span
Q11: In general, to calculate the time required
Q12: The isomerization of cyclopropane follows first order
Q13: For the reaction BrO<sub>3</sub><sup>-</sup> + 5Br<sup>-</sup>+