Multiple Choice
Use the graph of vapor pressure to determine the normal boiling point of CHCl3. 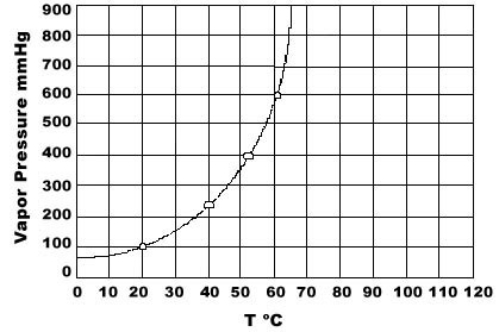
A) 19 C
B) 52 C
C) 60 C
D) 64 C
E) 70 C
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q125: How much energy (heat) is required
Q126: C<sub>8</sub>H<sub>18</sub> has a higher vapor pressure than
Q127: Octane, C<sub>8</sub>H<sub>18</sub>, boils at 125<sup> <span
Q128: NH<sub>3</sub> has a higher boiling point than
Q129: Which of the following constants is/are
Q131: CH<sub>4</sub> has a higher boiling point than
Q132: Acetic acid has a heat of fusion
Q133: C(CH<sub>3</sub>)<sub>4</sub> has a higher vapor pressure than
Q134: Which one of the following substances should
Q135: Which one of the following substances is