Multiple Choice
Use the graph of vapor pressure to determine the normal boiling point of O2. 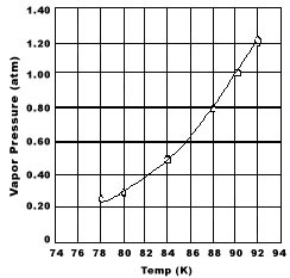
A) 84 K
B) 88 K
C) 90 K
D) 92 K
E) O2 doesn't boil because it is always a gas.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q85: Ethanol (C<sub>2</sub>H<sub>5</sub> - OH) will have a
Q86: The molar enthalpy of vaporization of
Q87: Given the following liquids and their
Q88: Which of the following phase changes is
Q89: An example of a covalent network solid
Q91: The shape of the water-to-glass meniscus results
Q92: The normal boiling point of bromine
Q93: Which one of the following substances will
Q94: NH<sub>3</sub> has a higher boiling point than
Q95: The intermolecular forces present in C<sub>6</sub>H<sub>6</sub> include