Multiple Choice
Polyethylene plastic consists of long chains of carbon atoms, each of which is also bonded to hydrogens as shown below: 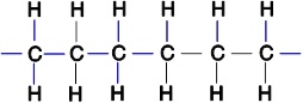 Water forms beads when placed on a polyethylene surface. Why
Water forms beads when placed on a polyethylene surface. Why
A) Water is nonpolar and polyethylene is nonpolar. Therefore there is very little interaction between these compounds. Accordingly, water beads are expected.
B) Water is highly polar and polyethylene is nonpolar. Therefore there is very little interaction between these compounds. Accordingly, water beads are expected.
C) Water is highly polar and polyethylene is polar. Therefore there is very little interaction between these compounds. Accordingly, water beads are expected.
D) Water is highly polar and polyethylene is ionic. Therefore there is very little interaction between these compounds. Accordingly, water beads are expected.
E) None of the above explains this example.
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Indicate all the types of intermolecular forces
Q3: Which type of intermolecular force is the
Q4: Which two properties are more typical of
Q5: The zincblende structure of ZnS has the
Q6: Which of the following properties is not
Q8: Arrange the following in order of increasing
Q9: Indicate all the types of intermolecular forces
Q10: The molar enthalpy of vaporization of
Q11: Given that the heat of vaporization
Q12: Which one of the following substances should