Multiple Choice
Ibuprofen is used as an analgesic for the relief of pain, and also to help reduce fever. What is the hybridization state of oxygen indicated by the arrow in the structure of ibuprofen shown below 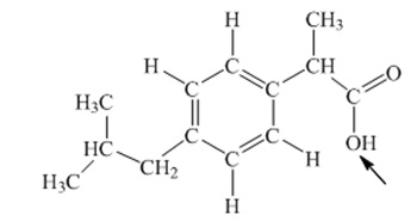
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q127: A molecule with 3 single bonds and
Q128: A molecule with 4 single bonds (and
Q129: According to the VSEPR theory, which one
Q130: According to the VSEPR theory, the molecular
Q131: A molecule with 4 single bonds and
Q133: A molecule with 2 single bonds and
Q134: What is the hybridization on the central
Q135: The bond angle in Cl<sub>2</sub>O is
Q136: A sp<sup>2</sup> hybridized central boron atom
Q137: Ibuprofen is used as an analgesic for