Multiple Choice
The number of pi bonds in the molecule below is 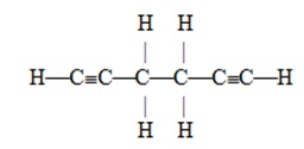
A) 2
B) 4
C) 6
D) 10
E) 15
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q68: Which one of the following molecules has
Q69: A sp<sup>2</sup> hybridized terminal oxygen atom
Q70: Which of the following correctly lists species
Q71: The bond angles in SF<sub>5</sub><sup>+</sup> are
Q72: Indicate the type of hybrid orbitals used
Q74: Two p<sub>y</sub> orbitals from two different atoms
Q75: Indicate the type of hybrid orbitals used
Q76: Give the number of lone pairs around
Q77: The geometry of the ClF<sub>3</sub> molecule is
Q78: There are no <span class="ql-formula"