True/False
Ibuprofen is used as an analgesic for the relief of pain, and also to help reduce fever. 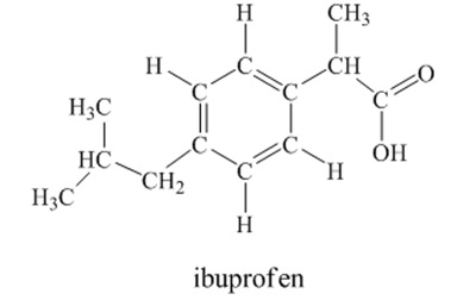 How many sigma bonds and pi bonds are contained in an ibuprofen molecule
How many sigma bonds and pi bonds are contained in an ibuprofen molecule
33 sigma bonds and 4 pi bonds
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q31: A bonding molecular orbital is of lower
Q32: The F - S - F
Q33: Ozone (O<sub>3</sub>) is an allotropic form of
Q34: The N - N - H
Q35: Which one of the following molecules is
Q37: N,N-diethyl-m-tolumide (DEET) is the active ingredient in
Q38: The BrF<sub>5</sub> molecule has polar bonds and
Q39: N,N-diethyl-m-tolumide (DEET) is the active ingredient in
Q40: Which one of the following molecules has
Q41: The geometry of the hybrid orbitals about