Multiple Choice
Shown here is the correct Lewis structure for PF5. According to VSEPR theory how many electrons are bonded to the P atom 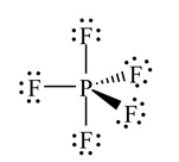
A) Four
B) Eight
C) Ten
D) Twelve
E) None of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q75: Indicate the type of hybrid orbitals used
Q76: Give the number of lone pairs around
Q77: The geometry of the ClF<sub>3</sub> molecule is
Q78: There are no <span class="ql-formula"
Q79: The bond angles in CO<sub>3</sub><sup>2-</sup> are
Q81: According to the VSEPR theory, the molecular
Q82: The electrons in the delocalized molecular orbitals
Q83: The number of pi bonds in the
Q84: Give the number of lone pairs around
Q85: The C-N-O bond angle in nitromethane,