Multiple Choice
The Lewis dot symbol for the chloride ion is
A) 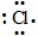
B) 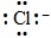
C)
D) 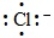
E) Cl-
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: BeF<sub>4</sub><sup>2-</sup> is called the fluoberyllate ion. The
Q4: List all types of bonding present in
Q5: Calculate the energy change for the
Q6: The Lewis structure reveals an unpaired electron
Q7: Use the Born-Haber cycle to calculate
Q9: The polarity of covalent bonds increases as
Q10: Which of the elements listed below would
Q11: Which response includes all the molecules below
Q12: Classify the Ca - Cl bond in
Q13: The Si - Cl bond has less