Multiple Choice
Carbonic acid, H2CO3, is a weak acid that contributes to the taste and produces the carbon dioxide bubbles in all carbonated beverages. How many valence electrons are used to show the Lewis structure for H2CO3 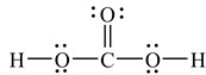
A) 24
B) 22
C) 20
D) 18
E) None of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q85: Covalent bonding is the only type of
Q86: Which one of the following is most
Q87: What is the formal charge on sulfur
Q88: Of the species NO<sub>2</sub>, NO, and N<sub>2</sub>,
Q89: Which of the elements listed below would
Q91: Which one of the following is most
Q92: Arrange the elements Ba, Br, and Ga
Q93: Classify the C - Cl bond in
Q94: Which of the following solids would have
Q95: Which one of the following compounds utilizes