Multiple Choice
Shown here is a Lewis structure for the chlorite ion, ClO2-, that obeys the octet rule, showing all non-zero formal charges. How many resonance structures for ClO2- that obey the octet are possible ?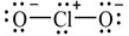
A) four
B) three
C) two
D) one
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q78: The total number of lone pairs in
Q79: Arrange the following bonds in order of
Q80: Which one of the following ionic solids
Q81: Classify the O - H bond in
Q82: The standard enthalpy of formation of
Q84: Shown here is a Lewis structure for
Q85: Covalent bonding is the only type of
Q86: Which one of the following is most
Q87: What is the formal charge on sulfur
Q88: Of the species NO<sub>2</sub>, NO, and N<sub>2</sub>,