True/False
Shown here is the Lewis structure for the chlorite ion, ClO2-, that expands the octet to minimize formal charge and if necessary places negative formal charges on the most electronegative atom(s). 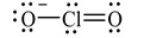
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q93: Classify the C - Cl bond in
Q94: Which of the following solids would have
Q95: Which one of the following compounds utilizes
Q96: Use the Born-Haber cycle to calculate
Q97: Assuming the octet rule is obeyed, how
Q99: The Lewis dot symbol for the
Q100: The total number of valence electrons in
Q101: The covalent bond with the greatest polarity
Q102: The azide ion, N<sub>3</sub><sup>-</sup>, is very reactive
Q103: Arrange the following bonds in order of