Multiple Choice
Shown here is a Lewis structure for the chlorate ion, ClO3-, that obeys the octet rule, showing all non-zero formal charges. How many resonance structures for ClO3- are possible that obey the octet rule 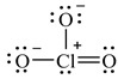
A) Four
B) Three
C) two
D) One
E) None of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q41: What is the formal charge on the
Q42: Assuming the octet rule is obeyed, how
Q43: Use the bond enthalpy data given
Q44: Which one of the following compounds utilizes
Q45: Which of the following ionic solids would
Q47: Which of the elements listed below is
Q48: For which of these species does the
Q49: The structure below depicts the correct Lewis
Q50: The number of lone electron pairs in
Q51: Use the bond enthalpy data given