Multiple Choice
Examine this Lewis structure for the phosphate ion, PO43-. How many valence electrons are around the P atom in this structure 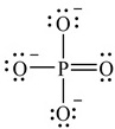
A) Eight
B) Four
C) Twelve
D) Nine
E) None of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q111: The number of lone electron pairs in
Q112: The total number of valence electrons in
Q113: The formal charge on the sulfur atom
Q114: Which of the following substances will display
Q115: Calculate the energy required for the
Q117: Which of the elements listed below would
Q118: The Lewis structure for CS<sub>2</sub> is:<br>A) <img
Q119: What type of chemical bond holds the
Q120: Of the following substances, KCl, KBr, and
Q121: The Lewis dot symbol for the S