Multiple Choice
The following Lewis structure depicts the product when boron trifluoride combines with ammonia. How many total valence electrons are shown in this structure 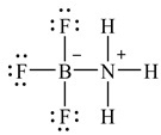
A) 28
B) 30
C) 32
D) 34
E) None of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q60: Arrange the elements C, O, and H
Q61: Which one of the following is most
Q62: Which one of the following compounds does
Q63: The number of resonance structures for the
Q64: Use bond energies to estimate the enthalpy
Q66: List all types of bonding present in
Q67: The Lewis structure reveals a double bond
Q68: How many covalent bonds will be drawn
Q69: Which one of the following is most
Q70: The following correctly depicts the Lewis dot