Multiple Choice
Determine the hybridization around each central atom. 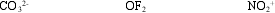
A) sp2 sp2 sp
B) sp2 sp3 sp
C) sp2 sp3 sp2
D) sp3 sp2 sp2
E) sp3 sp3 sp2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q20: In a molecule with triangular bipyramidal electron-pair
Q21: Use the VSEPR model to predict the
Q22: For which molecule will the electron-pair geometry
Q23: Which statement is false? A molecule may
Q24: Which of the following central atoms would
Q26: How many lone pairs of electrons does
Q27: When sketching a molecule, an atom connected
Q28: Use the VSEPR model to predict the
Q29: Which compound does not have tetrahedral electron-pair
Q30: Use the VSEPR model to predict