Multiple Choice
Determine the hybridization around each central atom. 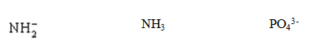
A) sp sp2 sp2
B) sp sp2 sp3
C) sp2 sp3 sp3d
D) sp2 sp3 sp3d2
E) sp3 sp3 sp3
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q29: Which compound does not have tetrahedral electron-pair
Q30: Use the VSEPR model to predict
Q31: Which interaction(s) exist(s) between CO molecules? <img
Q32: Use the VSEPR model to predict the
Q33: Because of London forces, molecules with _
Q35: Characterize the polarity of these molecules, in
Q36: According to the VSEPR model<br>A) The size
Q37: The carbon-carbon p bond in ethylene, CH<sub>2</sub>CH<sub>2</sub>,
Q38: Which substance experiences dipole-dipole interactions between its
Q39: Which description of a sigma bond is