Multiple Choice
Which of the molecules below undergo extensive hydrogen bonding? 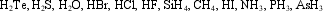
A) HBr, HCl, HF, H2O
B) H2O, HF, NH3
C) CH4, H2O, HF, NH3
D) H2S, H2O, HCl, HF
E) AsH3, NH3, HF, H2S
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: What is the major type of force
Q3: Which has the smallest bond angle?<br>A) <img
Q4: Which does not provide visual insight into
Q5: Which best describes the nature of the
Q6: Which statement about a central atom that
Q7: Which description of a pi bond is
Q8: What is the major type of force
Q9: London forces exist<br>A) for all molecules.<br>B) only
Q10: Use the VSEPR model to predict
Q11: Use the VSEPR model to predict