Multiple Choice
From the data below, calculate the approximate enthalpy change for the reaction below. 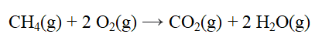

A) -806 kJ
B) -98 kJ
C) 98 kJ
D) 120 kJ
E) 806 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: How many electrons will be in the
Q12: What is the formal charge on sulfur
Q13: Which statement about cis and trans isomers
Q14: Assume all hydrocarbons given are linear. Which
Q15: Which of the following compound can exhibit
Q17: Which bond is least polar?<br>A) C-C<br>B) C-N<br>C)
Q18: Determine the number of electrons required by
Q19: Write the correct Lewis dot structure for
Q20: Which molecule contains a triple bond?<br>A) C<sub>2</sub>H<sub>4</sub><br>B)
Q21: Which bond is shortest?<br>A) carbon-oxygen double bond<br>B)