Multiple Choice
From the data below, calculate the approximate enthalpy change of reaction for the reaction below. 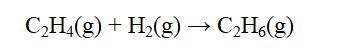

A) -392 kJ
B) -219 kJ
C) -128 kJ
D) 219 kJ
E) 392 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q57: Write the correct Lewis structure for AsCl<sub>5</sub>.
Q58: Which element is the most electronegative?<br>A) phosphorus<br>B)
Q59: Oxygen atoms contribute _ electrons to a
Q60: Predict the relative bond lengths of the
Q61: Which statements about resonance are true?<br>I. Resonance
Q62: Which bond is shortest?<br>A) carbon-oxygen single bond<br>B)
Q63: Which statement about molecular orbital theory is
Q64: The cyanide ion, CN<sup>-</sup>, has a(n) _
Q65: An element is allowed to have an
Q67: Which statement properly describes the formal charges