Multiple Choice
Which statement properly describes the formal charges on the atoms in 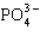 ?
?
A) -2 on oxygen, +5 on phosphorus
B) -1 on oxygen, +4 on phosphorus
C) -1 on oxygen, +1 on phosphorus
D) +1 on oxygen, -1 on phosphorus
E) +1 on oxygen, -3 on phosphorus
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: Write the correct Lewis dot structure for
Q4: How many electrons will be in the
Q5: Write the singly bonded Lewis dot structure
Q6: Write the correct Lewis dot structures for
Q7: List these compounds in order of decreasing
Q9: Predict qualitatively the relative bond lengths of
Q10: Benzene is an example of a resonance
Q11: How many electrons will be in the
Q12: What is the formal charge on sulfur
Q13: Which statement about cis and trans isomers