Multiple Choice
Arrange these elements in order from largest to smallest atomic radii. 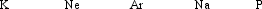
A) Ar > K > Na > Ne > P
B) K > Ar > P > Na > Ne
C) Ar > P > Na > Ne > K
D) Ne > Ar > P > Na > K
E) K > Na > P > Ar > Ne
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q43: Which halogen has the most negative electron
Q44: Which statement about the formation of solid
Q45: What is the correct electron configuration for
Q46: _ is the number of peaks or
Q47: What is a major shortcoming of the
Q49: How many electrons can the second principal
Q50: When n = 3, l can equal
Q51: Which statement regarding atomic orbitals is false?<br>A)
Q52: What is the electron configuration of Br<sup>-</sup>?<br>A)
Q53: Which element has the largest atomic radius?<br>A)