Multiple Choice
Arrange these elements in order of increasing ionization energy. 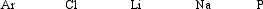
A) P < Cl < Ar < Li < Na
B) Na < Li < P < Cl < Ar
C) Ar < Cl < Na < Li < P
D) Cl < Ar < Na < Li < P
E) P < Cl < Ar < Na < Li
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q32: Which word or phrase least applies to
Q33: Light has a frequency of 7.21 ×
Q34: What is the electron configuration of Li<sup>+</sup>?<br>A)
Q35: Which statement is true?<br>A) The 3d orbitals
Q36: Which set of quantum numbers is not
Q38: Which element has the largest atomic radius?<br>A)
Q39: Which statement about light is true?<br>A) Light
Q40: What is the electron configuration of O<sub>2</sub><sup>-</sup>?<br>A)
Q41: Which word or phrase least applies to
Q42: As one moves closer to the nucleus,