Multiple Choice
Determine the heat of reaction for the process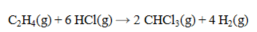
using the information given below:
A) -295.3 kJ
B) -29.2 kJ
C) +29.2 kJ
D) +295.3 kJ
E) +398.4 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: A 0.100 mole sample of CH<sub>4</sub> reacts
Q2: The quantity of energy required to increase
Q3: Which of the following is an example
Q4: The temperature of a 21.6 g sample
Q6: What is the enthalpy change when 175
Q7: In a(n) _ reaction, the energy of
Q8: Determine the quantity of ice required to
Q9: The First Law of Thermodynamics states that:<br>A)
Q10: Which of the following is not an
Q11: Determine the heat of reaction for the