Multiple Choice
Determine the heat of reaction for the process 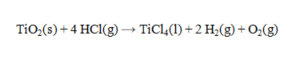
using the information given below:
A) -320.1 kJ
B) -233.7 kJ
C) 233.7 kJ
D) 320.1 kJ
E) 504.7 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q41: Which term refers to a quantity of
Q42: A bomb calorimeter has a heat capacity
Q43: Heating a 25.0 g sample of each
Q44: How many kilojoules are there in 150
Q45: The standard enthalpies of formation for several
Q47: Determine if each of the four situations
Q48: According to the First Law of Thermodynamics,
Q49: Enthalpy change is equal to heat transfer
Q50: Determine the incorrect relationship given below.<br>A) 1
Q51: Match the following: