Multiple Choice
Which statement about the reaction below is true, given large amounts of reactants? 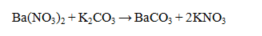
A) Ba(NO3) 2 is insoluble in water and will precipitate.
B) K2CO3 is insoluble in water and will precipitate.
C) BaCO3 is insoluble in water and will precipitate.
D) Both BaCO3 and KNO3 are insoluble in water and will precipitate.
E) All compounds in the reaction are soluble in water and no reaction occurs.
Correct Answer:

Verified
Correct Answer:
Verified
Q25: What is oxidized in the reaction below?
Q26: A neutralization reaction involves the reaction of
Q27: How many moles of NaOH are present
Q28: A 25.0 mL solution of HNO<sub>3</sub> is
Q29: Which of the following represents oxidation?<br>A) 2
Q31: What is the oxidation number of O
Q32: What is the correct formula for the
Q33: Which of the following factors cause exchange
Q34: Molarity is a unit of solution concentration
Q35: A solution is prepared by dissolving 20.0