Multiple Choice
Ammonia and sulfuric acid react according to the equation given below. How many milliliters of 0.110 M sulfuric acid are required to exactly neutralize 25.0 mL of 0.0840 M NH3 solution? 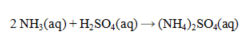
A) 1.46 mL
B) 1.82 mL
C) 3.64 mL
D) 5.85 mL
E) 9.55 mL
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q38: A solution is made by dissolving 15.0
Q39: What is the correct formula for the
Q40: A 25.00 mL sample of HCl solution
Q41: What is the oxidation number of P
Q42: Which of the following represents reduction?<br>A) Al
Q44: If a 45.0 mL sample of 2.20
Q45: A chemical reaction for which spectator ions
Q46: Which compound will not dissolve in water
Q47: A solution is made by dissolving 60.0
Q48: A 25.00 mL sample of HCl solution