Multiple Choice
The Roman numerals in the reaction given represent the coefficients in the balanced chemical equation. What are the values of the coefficients? 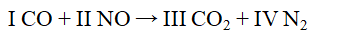
A) 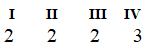
B) 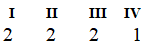
C) 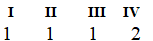
D) 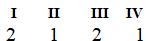
E) 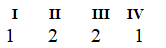
Correct Answer:

Verified
Correct Answer:
Verified
Q10: How many grams of TiCl<sub>4</sub> are needed
Q11: Classify the following reaction. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="Classify
Q12: The Roman numerals in the reaction given
Q13: What is the theoretical yield (in grams
Q14: In the reaction given below, how many
Q16: How many grams of KClO<sub>3</sub> are needed
Q17: Classify the following reaction. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="Classify
Q18: The complete combustion of a hydrocarbon produces
Q19: How many moles of O<sub>2</sub> will be
Q20: Classify the following reaction. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="Classify