Multiple Choice
In the reaction below, 8.0 g of H2 react with 7.0 g of O2. Which of the following statements is incorrect? 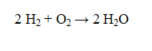
A) More moles of hydrogen are consumed than moles of oxygen.
B) More grams of hydrogen are consumed than grams of oxygen.
C) More grams of oxygen are consumed than grams of hydrogen.
D) More moles of water are produced than moles of oxygen are consumed.
E) More grams of water are produced than grams of oxygen are consumed.
Correct Answer:

Verified
Correct Answer:
Verified
Q47: How many grams of Na<sub>2</sub>O are formed
Q48: What is the percent yield when 0.750
Q49: A reaction has a theoretical yield of
Q50: In the reaction given below, if 12
Q51: A combination reaction is considered the opposite
Q53: Which of the following cannot be determined
Q54: How many moles of oxygen will be
Q55: The Roman numerals in the reaction given
Q56: If 935 g of cesium reacts with
Q57: Which statement about the expression 6 C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>